Figure 12 shows two identical containers A and B into which a copper rod is fitted. The containers are well-lagged.
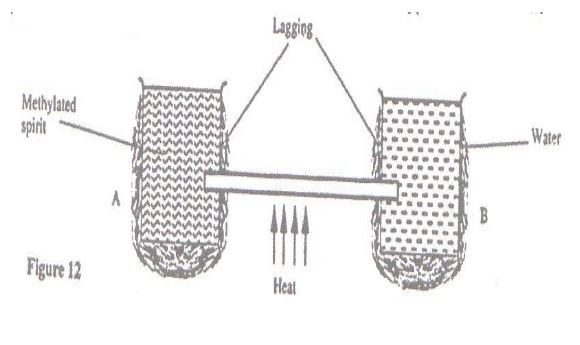
The liquids in the containers were initially at the same temperature. If the heat is applied continuously at the position shown,state with a reason the container through which the loss of heat is likely to be higher.
Answer:
- Heat loss in A is higher. Methylated spirit being more volatile evaporates
causing loss of heat of vaporization.
causing loss of heat of vaporization.
Share To Friends Via:



 The control grids in a Cathode Ray Oscilloscope (CRO) is used to control the brightness of the beam on the screen. How is this achieved?
The control grids in a Cathode Ray Oscilloscope (CRO) is used to control the brightness of the beam on the screen. How is this achieved?