In an experiment, carbon (IV) oxide gas was passed over heated charcoal
and the gas produced collected as shown in the diagram below.
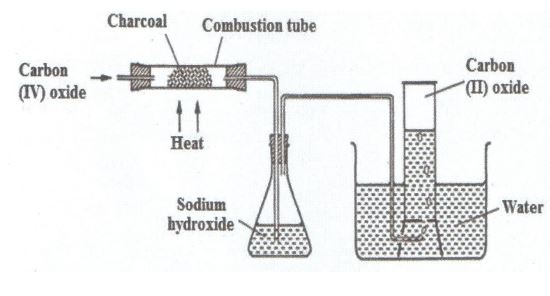
i) Write an equation for the reaction that took place in the combustion tube.
(ii) Name another substance that can be used instead of sodium hydroxide.
(iii) Describe a simple chemical test that can be used to distinguish
between carbon (IV) oxide and carbon (II) oxide.
iv) Give one use of carbon (II) oxide.
Answer:
i) #C(s)+CO_2(g) rightarrow2CO(g)#
ii)- Potassium hydroxide.
iii)- Pass the gas through lime water (carbon (IV) oxide).
form a white precipitate but carbon (II) oxide does not give precipitate.
iv)- Used in manufacture of methane.
- Used in extraction of metals as a reducing agent.
ii)- Potassium hydroxide.
iii)- Pass the gas through lime water (carbon (IV) oxide).
form a white precipitate but carbon (II) oxide does not give precipitate.
iv)- Used in manufacture of methane.
- Used in extraction of metals as a reducing agent.
Share To Friends Via:



 in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III
in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III