The apparatus shown below was used to investigate the effect of carbon (II) oxide on copper (II) oxide.
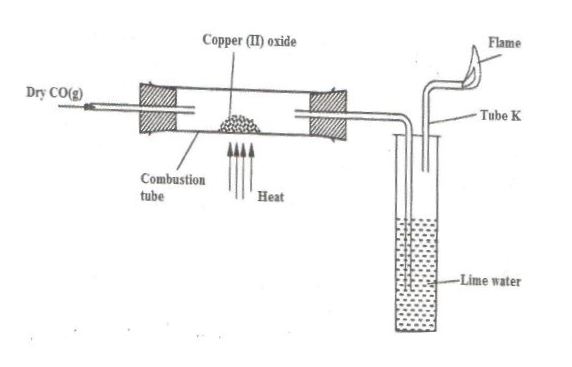
(a) State the observation that was made in the combustion tube at the end of the experiment.
(b) Write an equation for the reaction that took place in the combustion tube.
(c) Why is it necessary to burn the gas coming out of tube K?
Answer:
a)- Copper (II) oxide turned from black to brown.
b)#CuO(s)+CO(g)rightarrowCu(s)+CO_2(g)#
c)- It is poisonous hence should not be released into the air.
b)#CuO(s)+CO(g)rightarrowCu(s)+CO_2(g)#
c)- It is poisonous hence should not be released into the air.
Share To Friends Via:



 in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III
in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III