a) The set-up below was used to collect gas F, produced by the reaction between water and calcium metal
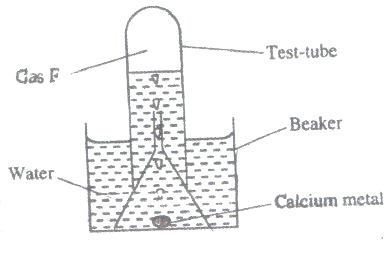
(i) Name gas F
ii) At the end of the experiment, the solution in the beaker was found to be weak base. Explain why solution was a weak base.
iii) Give one laboratory use of the solution formed in a beaker.
(b) The scheme below shows some reactions starting calcium oxide. Study it and answer questions that follow.
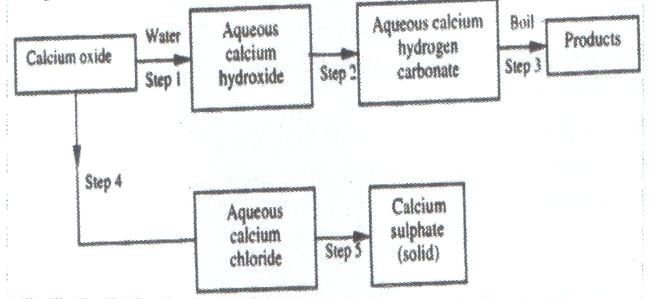
(i) Name the reagents used in step 2 and 4
(ii) Write an equation for the reaction in step 3
(iii) Describe how a solid sample of anhydrous calcium sulphate is obtained in step 5
Answer:
a) i)Hydrogen gas
ii)- Calcium hydroxide (Ca#(OH)_2#) formed is slightly soluble in water. Therefore only a few OH- are produced in solution
iii)- It is used for testing presence of carbon (IV) oxide (#CO_2#).
b) i)
- Step 2-carbon (IV) oxide)
- Step 4-dilute hydrochloric acid
ii)#Ca(HCO_3)_2(aq) rightarrowCaCO_3(s)+#
#CO_2(g)+H_2O(g)#
iii)- Add an aqueous solution of sulphuric (VI) acid. Precipitates of
calcium sulphate forms. Filter to obtain calcium sulphate as a residue
ii)- Calcium hydroxide (Ca#(OH)_2#) formed is slightly soluble in water. Therefore only a few OH- are produced in solution
iii)- It is used for testing presence of carbon (IV) oxide (#CO_2#).
b) i)
- Step 2-carbon (IV) oxide)
- Step 4-dilute hydrochloric acid
ii)#Ca(HCO_3)_2(aq) rightarrowCaCO_3(s)+#
#CO_2(g)+H_2O(g)#
iii)- Add an aqueous solution of sulphuric (VI) acid. Precipitates of
calcium sulphate forms. Filter to obtain calcium sulphate as a residue
Share To Friends Via:



 in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III
in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III