The diagram below represents part of a set-up used to prepare and collect gas T.
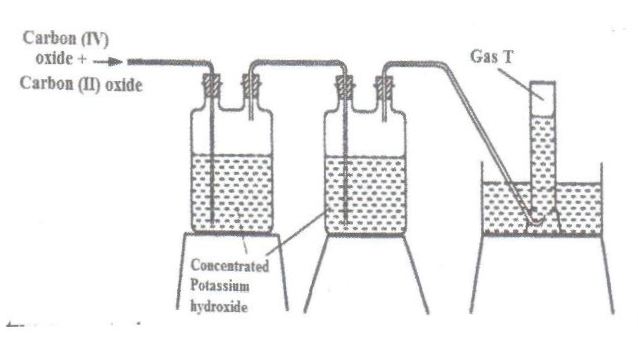
(a) Name two reagents that are reacted to produce both carbon (IV) oxide and carbon (II) oxide.
(b) Write the equation for the reaction which takes place in the wash bottles.
(c) Give a reason why carbon (II) oxide is not easily detected.
Answer:
a)Oxalic acid and concentrated sulphuric (VI) acid.
b)
#2KOH(aq)+CO_2(g)rightarrow#
#K_2CO_3(aq)+H_2O(l)#
c)
-It is odourless, colourless.
b)
#2KOH(aq)+CO_2(g)rightarrow#
#K_2CO_3(aq)+H_2O(l)#
c)
-It is odourless, colourless.
Share To Friends Via:



 in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III
in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III