Study the flow chart below and answer the questions that follow.
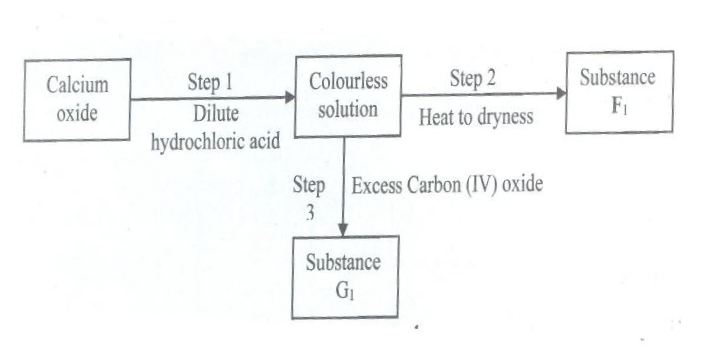
(a) Give the name of the process that takes place in step I
(b) Give ;
(i) The name of substance G1
(ii) One use of substance F1
Answer:
a)
- Neutralization
b) i)
- Calcium hydrogen carbonate
ii)- Drying agent
- Neutralization
b) i)
- Calcium hydrogen carbonate
ii)- Drying agent
Share To Friends Via:



 in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III
in an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of the soap solutions required to form lather with #1000cm^3# of each sample of water before and after boiling.
a) Which water sample is likely to be soft? Explain.
(b) Name the cause of change in the volume of soap solution used in sample III